What Are the Common Corrosion Types of Stainless Steel Plates?
There are six common types of stainless steel plate corrosion. Keep reading to find out which type may cause corrosion on your stainless steel plate.
1. General Corrosion
This type of corrosion usually occurs evenly across the entire stainless steel plate surface. The passive layer may be attacked uniformly depending on the concentration and temperature of hydrochloric and sulphuric acids and the metal loss is distributed over the entire surface of the steel. Although general corrosion reduces the effective stress-bearing area and service life of the SS plate, it is less harmful than local corrosion.
2. Galvanic Corrosion
If two dissimilar metals are welded together—whether by accident or by design. Corrosion occurs when two metals with different properties are connected via a common electrolytic material (such as water or weld filler material), there may be a flow of electrical current from one material to the other. This will cause the less “noble” metal (meaning the metal that more readily accepts new electrons) to become an “anode” and start to corrode more quickly.
The speed of this corrosion will change depending on a few factors, such as the specific types of stainless steel being joined, what kind of welding filler was used, ambient temperature and humidity, and the total surface area of the metals that are in contact with one another.
The best preventative measure for bimetallic corrosion is to avoid joining two dissimilar metals permanently in the first place. A close second is to add a coating to the metals to seal them off with a coating to prevent the flow of electrons from the cathode to the anode.
It should also be noted that using a weld filler that is too dissimilar to the metals being joined can also result in galvanic corrosion at the weld site as well as carbon steel screws and bolts being used to join stainless steel.

3. Intergranular Corrosion
The most typical example is intergranular corrosion in the welding area: the metal weld begins to crack and appears like cracks.
When austenite is heated to 450-900°C during welding, the chromium in the intergranules easily precipitates together with carbon to form chromium carbide. Due to the great affinity between carbon and chromium, it takes up 17 times the carbon content of chromium to form carbides, thereby significantly reducing the corrosion resistance of stainless steel. To achieve corrosion resistance, the carbon content in stainless steel must be controlled. The manufacturer can screen out materials with a carbon content of up to 0.02% and put them into production, which can greatly improve the ability to resist intergranular corrosion. In addition, stainless steel plates such as 321 grade can also be added with titanium (niobium), which has a stronger affinity for carbon than chromium, to enhance the stability of the chromium element and thus improve the resistance to intergranular corrosion.
4. Pitting Corrosion
It’s a localized type of corrosion that leaves cavities or holes. The passive layer on the stainless steel plate can be attacked by certain chemical species. Therefore, pitting corrosion can occur when stainless steel plate is exposed to chloride-rich environments such as salt or bench. For example, stainless steel plates used in cargo ships experience pitting over time, which is a result of being in constant contact with seawater and sea breezes — both of which contain high levels of salt.
In addition to chloride corrosion, pitting corrosion can also be caused by elevated temperatures for extended amounts of time or a lack of oxygen to the surface.
To avoid pitting corrosion, it’s important to use stainless steel plate that does not come into prolonged contact with harmful chemicals or by choosing a grade of steel that is more resistant to chloride attack—such as grade 316 stainless steel. Avoid using grades that are known for their weak resistance to chlorides —like 304 stainless steel. Alternatively, a specialized coating can be applied to the steel surface to prevent direct contact with chlorides in the environment.
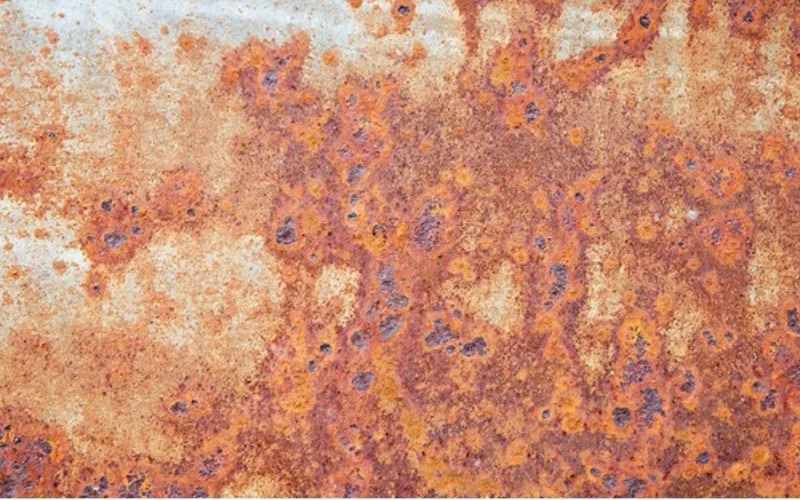
5. Crevice Corrosion
It is a kind of localized corrosion, occurring at the crevice between two joining surfaces of two metals or a metal and a non-metal.
Stainless steel plate requires a supply of oxygen to make sure that the passive layer can form on the surface. For example, when two stainless steel plates are bolted together and in contact with electrolyte solutions, It will firmly adsorb the plates and repel oxygen, thus starting corroding between gaps. If the electrolyte is sodium chloride and is heated, the corrosion process becomes significantly faster.
Crevice corrosion is avoided by sealing crevices with a flexible sealant or by using a more corrosion-resistant grade.
This can be prevented by sealing crevices in your stainless steel material with flexible sealant. Using proper welding techniques and ensuring drainage can also prevent the creation of additional crevices.
6. Stress Corrosion
Stress corrosion is a relatively rare form of corrosion, which requires a specific combination of tensile stress, temperature, and corrosive species, often the chloride ion, for it to occur. One reason is that the inner surfaces of most high-temperature equipment still have some residual internal stress after processing and manufacturing. If there is only tensile stress but no compressive stress inside the stainless steel plate, then corrosion cracking will occur with the presence of stress. If it does happen, it can be rapid, breaking down the mechanical properties of stainless steel plate in days rather than months or years. Another form known as sulfide stress corrosion cracking (SSCC) is associated with hydrogen sulfide in oil and gas exploration and production.
For mechanical construction, it is impossible to eliminate tensile stress. Therefore, in actual operation, it is very important to control the critical temperature. Eliminating or not using high-temperature printing and dyeing auxiliaries that generate chloride ions is an important measure to prevent stress corrosion cracks.

7. Chemical Corrosion
Firstly, the oil, dust, acid, alkali, salt, etc. attached to the surface of the stainless steel plate workpiece are converted into corrosive media under certain conditions and chemically react with certain components in the stainless steel plate to cause chemical corrosion and rust. Secondly, the damage to the passivation film by various scratches will reduce the protective performance of the stainless steel plate, and it will easily react with chemical media, causing chemical corrosion and rust. Finally, improper cleaning after pickling and passivation will cause residual liquid to remain, which will also cause direct chemical corrosion to the stainless steel plate.
8. Electrochemical Corrosion
When two different metals come into contact and invade the electrolyte solution, the less inert metal becomes the anode, and the more inert metal becomes the cathode, and the anode metal will continue to produce ions and move towards the cathode, causing the anode metal itself to Corroded. That will produce electrochemical corrosion. The main forms of electrochemical corrosion are as follows:
1. Carbon steel pollution: Electrochemical corrosion is caused by the scratches caused by the contact between stainless steel plates and carbon steel parts and the corrosive medium forming a galvanic cell.
2. Cutting: The adhesion of cutting slag, splash, and other rust-prone substances and corrosive media form galvanic cells, resulting in electrochemical corrosion.
3. Baking: The composition and metallographic structure of the flame heating area change unevenly, and form a galvanic cell with the corrosive medium to produce electrochemical corrosion.
4. Welding: Physical defects in the welding area (undercuts, pores, cracks, lack of fusion, lack of penetration, etc.) and chemical defects (coarse grains, poor chromium at grain boundaries, segregation, etc.) and corrosive media from galvanic cells to produce electricity, thus forming electrochemical corrosion.
5. Material: The chemical defects (uneven composition, S, P impurities, etc.) and surface physical defects (porosity, blisters, cracks, etc.) of stainless steel are conducive to forming a galvanic cell with the corrosive medium and causing electrochemical corrosion.
6. Passivation: The poor passivation effect of pickling results in uneven or thin passivation film on the surface of stainless steel plate, which is prone to electrochemical corrosion.
7. Cleaning: The remaining pickling passivation residue and the chemical corrosion products of stainless steel plate will form electrochemical corrosion on stainless steel parts.

9. Atmospheric Corrosion
Although stainless steel plate is highly corrosion resistant, it is prone to rust in certain environments, especially in areas with high air humidity, continuous rainy weather, or environments with high pH in the air. Therefore, it is important to place the stainless steel plate in a dry and ventilated environment.
10. Corrosion Caused by Temperature Extremes
Stainless steel is designed to have a high melting point (usually above 600˚C). While it can withstand temperature extremes without melting, it may experience other changes that affect its ability to resist corrosion. One common example is when stainless steel plates are exposed to high temperatures (such as those used in many heat treatment/annealing processes) and form scales. When scales form on hot metal, the flaky leftover material can cause bimetallic corrosion since the scales have a different composition from the base metal.
Additionally, temperature extremes can also cause exposed stainless steel plate to lose their protective oxide layer for some time. This layer will take some time to reform after being stripped away by the heat. Without this layer, the risk of corrosion increases.
To prevent this from happening, it’s important to check the recommended operating temperatures for any given stainless steel plate to see if the temperatures used in your manufacturing processes exceed those limits. If the temperatures in your project or operations exceed those limits, consider adjusting temperatures or procuring a grade of stainless steel that better matches your needs.
11. Corrosion Caused by the Smelting Process
Large stainless steel plants with good smelting technology, advanced equipment, and advanced processes can ensure the control of alloy elements, removal of impurities, and control of billet cooling temperature. Therefore, the quality of their stainless steel products is stable and reliable, and they are not easy to rust. On the contrary, some small steel plants have backward equipment and backward processes. During the smelting process, impurities cannot be removed, and the products produced will inevitably rust. Therefore, when buying stainless steel plates or other stainless steel products, remember to choose a reliable and powerful stainless steel plate manufacturer and supplier.

Get Your Stainless Steel Plate from Gnee Steel
Preserving the quality of your stainless steel plate products is all about knowing what can affect the material’s integrity and natural resistance properties. Knowing what corrodes stainless steel will help you keep your products structurally sound and usable for as long as possible.
For those wanting only the best quality metals, knowing additional prevention measures as well as receiving professional insights is recommended. Contact Gnee Steel today to consult experts on ideal fit and safety precautions for your stainless steel products!


